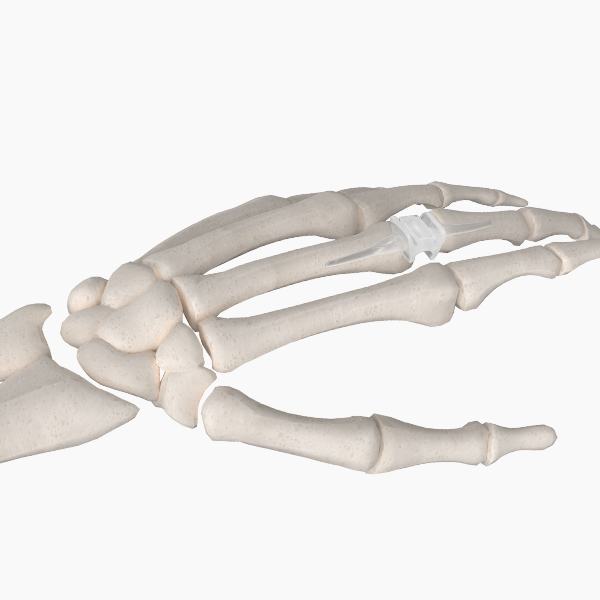
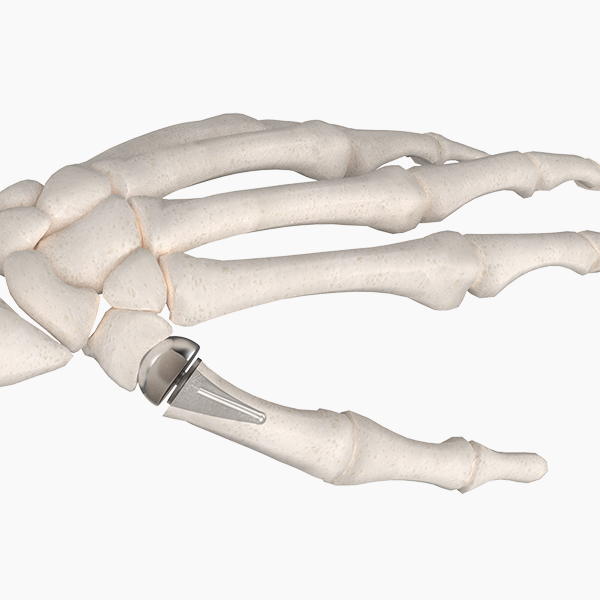
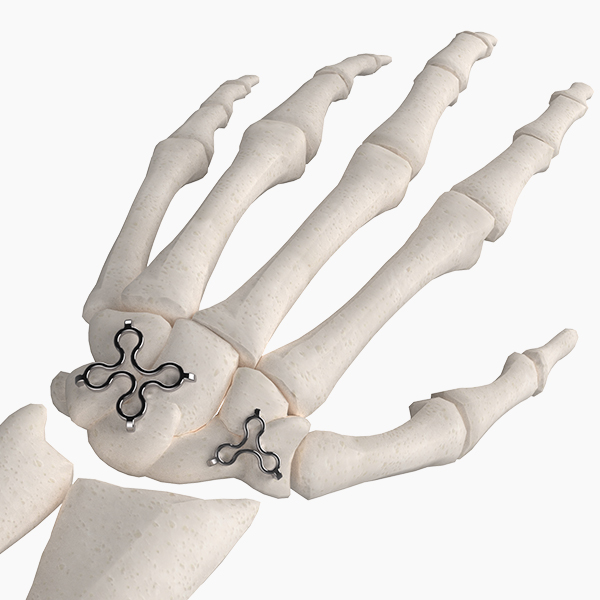
Our unique Hand & Wrist portfolio offers proven joint replacements and fixation devices to address common conditions and procedures.
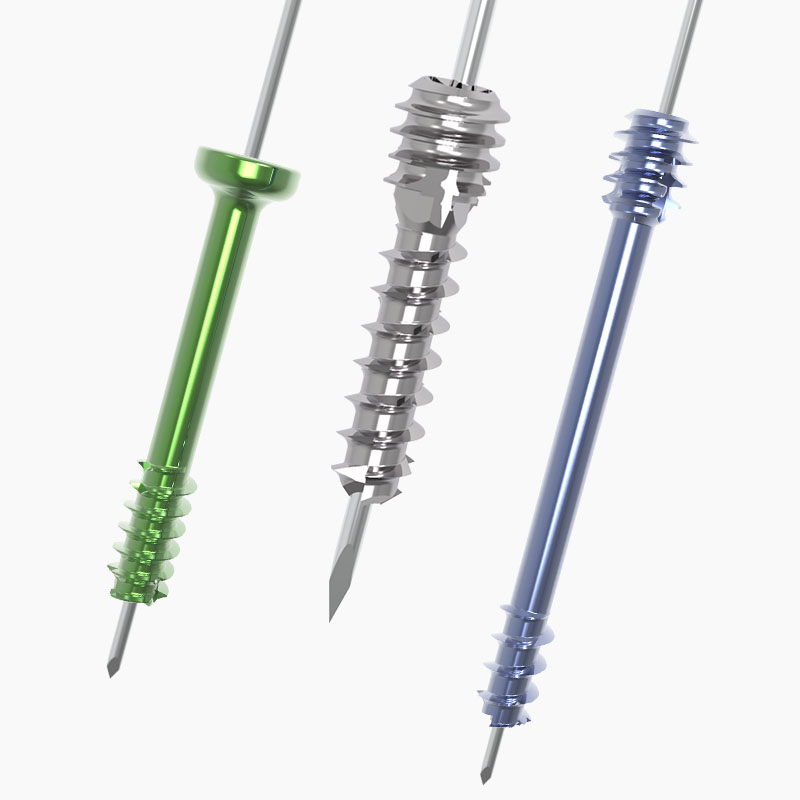
Shark Screw
Jason Pringle2025-08-22T11:47:47+00:00Shark Screw® is a screw made from human donor bone and can be used in orthopedics and trauma surgery.