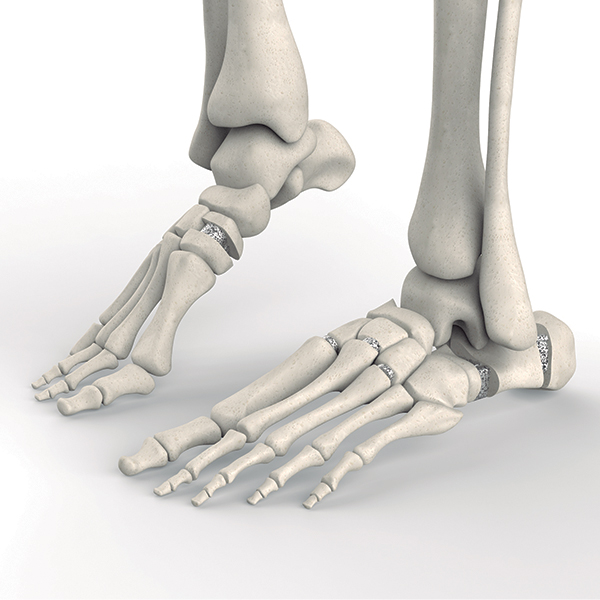
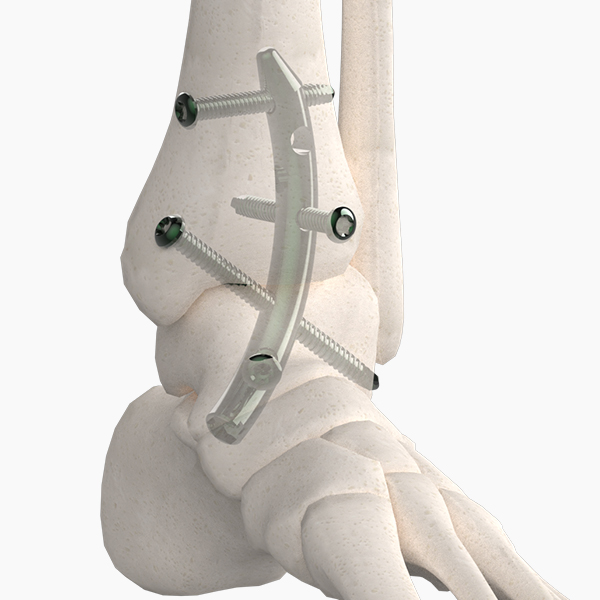
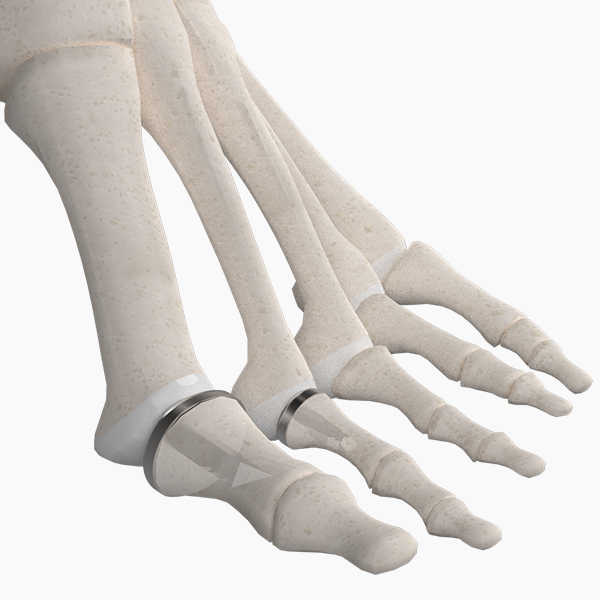
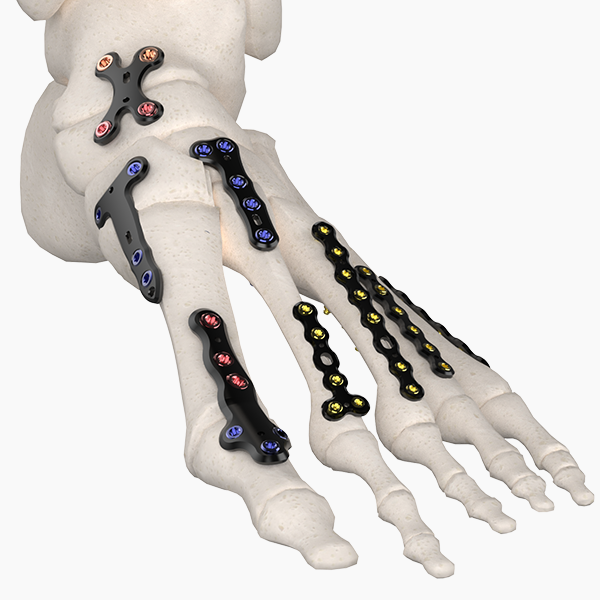
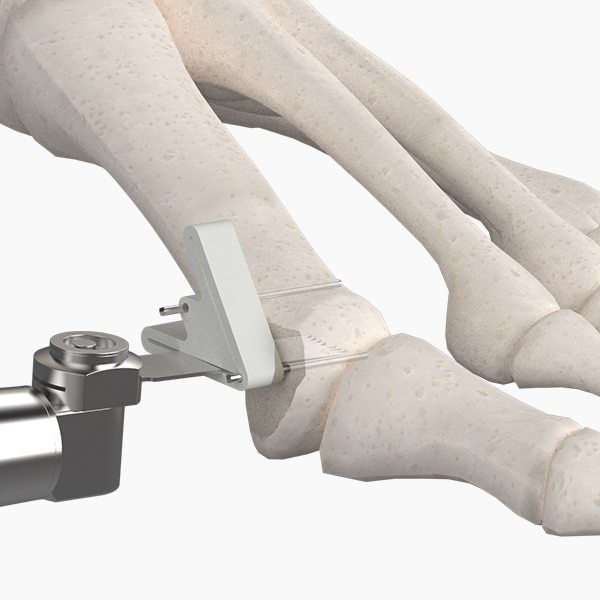
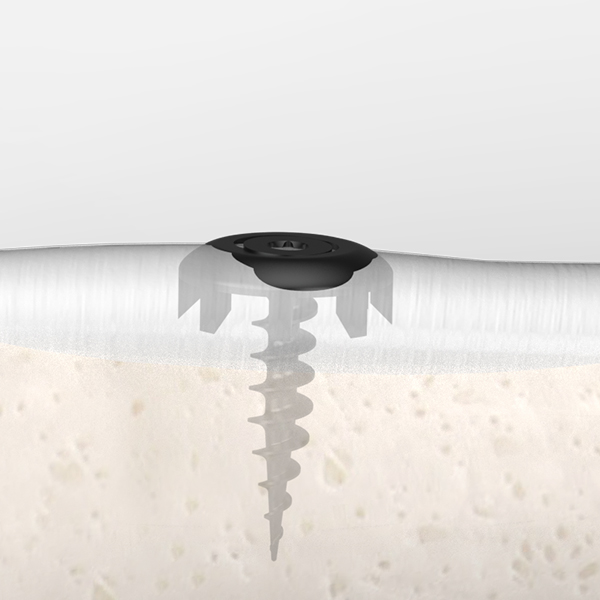
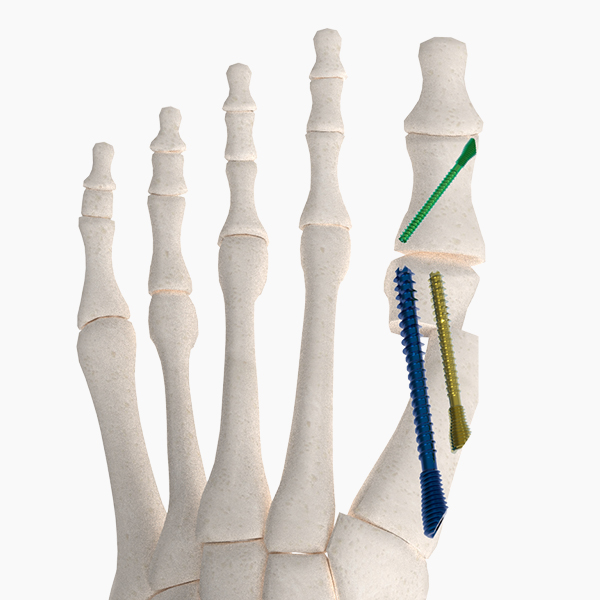
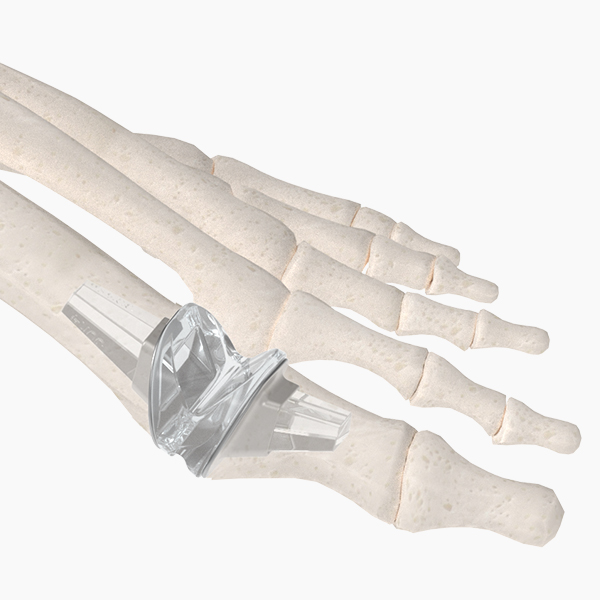
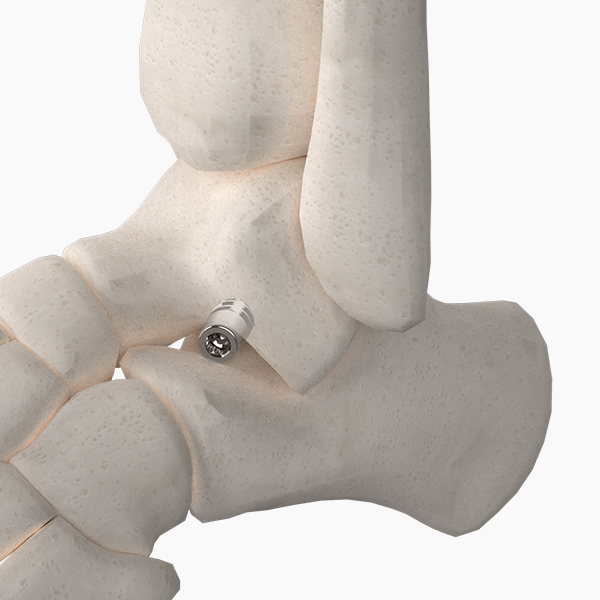
OsteoSinter® EVANS and COTTON Wedges are porous titanium implants engineered for use in the surgical correction of adult-acquired flatfoot deformities.
Shark Screw
Jason Pringle2025-08-22T11:47:47+00:00Shark Screw® is a screw made from human donor bone and can be used in orthopedics and trauma surgery.
Shotel™ Ankle Arthrodesis Nail System
Jason Pringle2026-02-13T17:41:52+00:00The Shotel™ Ankle Arthrodesis Nail System is an intramedullary (IM) nail intended to provide compression and rigid fixation for primary ankle fusions. The unique curved design allows an approach through the medial side of the talus and is designed to achieve fusion at the tibiotalar joint while allowing unrestricted motion to remain at all other joints.
MPJ Hemi Implants
bioproimplants_admin2026-02-13T17:54:48+00:00The BioPro® MPJ Hemi Implant is the only Hemi backed by 70+ years of clinical data. Our low-profile, press-fit implants are designed to replace the articular surface of the proximal phalanx in a painful, arthritic metatarsophalangeal (MTP) joint.
Comprehensive Foot Plating System
Jason Pringle2026-02-13T17:50:57+00:00A comprehensive plating system merges modern technology with plating basics. The system includes 45 universal plates as well as indication-specific plating families. The plates feature MVA (multiple variable angle) locking technology that allows screws to lock into the plate up to 25 degrees.
Accu-Cut Osteotomy Guide System
bioproimplants_admin2026-02-13T17:59:23+00:00The Accu-Cut Osteotomy Guide System is a disposable, sterile packaged system that includes a guide, two universal saw blades and two 0.045 double trocar K-wires. Using an Accu-Cut Guide in conjunction with your preferred osteotomy ensures precision with all cuts for optimal bone contact and improved fusion success.
Tendon Anchor System
bioproimplants_admin2026-02-13T17:52:48+00:00The BioPro Tendon Anchor System (T.A.S.) is a toothed, titanium anchor designed for soft tissue reattachment to bone. The sutureless design simplifies soft tissue reattachment and allows for increased pull-out strength.
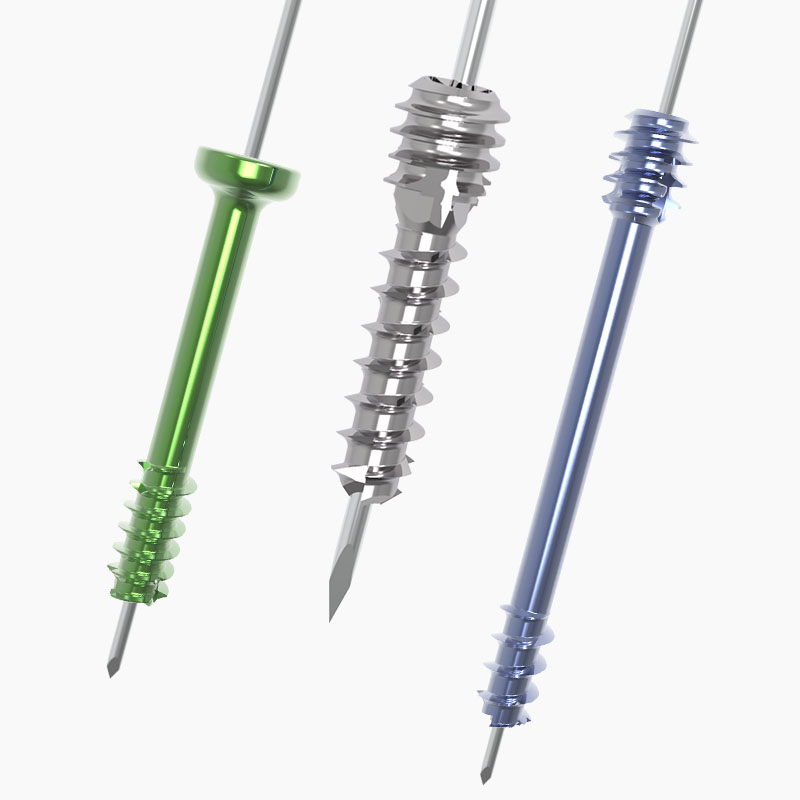
Tool VIP Screws
Jason Pringle2026-02-13T17:57:28+00:00The TOOL VIP Screw System offers three different diameter screws and specialized instrumentation designed for minimally invasive surgery. The neutral pitch allows surgeons to maintain length of the osteotomy site and optimize stability.
Silktoe
Jason Pringle2026-02-13T17:55:26+00:00The BRM Silktoe® Implant is a new generation flexible silicone spacer for first metatarsophalangeal (1st MTP) joint arthroplasty.
Digital Compression Screw
bioproimplants_admin2026-02-13T17:56:20+00:00The Digital Compression Screw (D.C.S.) is a solid, stainless steel screw specifically designed to address digital fusions. The 1.5mm and 1.8mm diameters range from 20mm to 55mm in length, allowing for fusion of the DIPJ, PIPJ, or both.
Horizon Subtalar
bioproimplants_admin2026-02-13T17:57:02+00:00The BioPro Horizon Subtalar Implant is used for the treatment of flatfoot and posterior tibial tendon dysfunction in both children and adults. Implanted within the sinus tarsi, it is designed to block excessive pronation while allowing normal subtalar joint motion.
Memory Staple
bioproimplants_admin2026-02-13T17:58:13+00:00The Memory Staple is a nitinol memory-alloy staple designed to provide fast and stable fixation in a variety of procedures. The staple provides dynamic compression design to facilitate bone healing.
Cannulated Compression Screws
Jason Pringle2026-02-13T17:58:42+00:00Go-EZ Headed Cannulated Screws and HBS Headless Cannulated Screws are manufactured from implant grade Titanium and available in multiple diameters and lengths. Each screw is individually sterile packed and housed in a protective blister to protect screw threads. This ensures sharp cutting teeth and improves traceability for hospital and patient records.
K-Wires
Jason Pringle2025-07-23T18:32:54+00:00Our medical grade stainless steel Kirschner wires (k-wires) are available in 0.9mm (.035″), 1.1mm (.045″), 1.4mm (.054″), and 1.6mm (.062″) diameters. We offer the k-wires as individually sterile packaged or in non-sterile multi-packs.
Fasciotome
Jason Pringle2025-07-03T17:51:23+00:00The BioPro Fasciotome™ is intended to assist surgeons in percutaneous tenotomies and plantar fasciotomies. The Fasciotome features an extremely sharp, one-sided blade for effortless releases; provided in convenient single-use, sterile packs.